Enzyme That Adds Phosphate, or kinases, are crucial players in cellular processes. These enzymes catalyze the transfer of a phosphate group from ATP to a substrate, a process known as phosphorylation. This seemingly simple reaction profoundly impacts a vast array of biological functions, from cellular signaling and metabolism to gene expression and cell growth. Understanding kinases is key to comprehending life’s intricate mechanisms and their dysregulation in disease.
This exploration delves into the mechanisms of kinase action, highlighting the diverse roles of these enzymes across various biological pathways. We’ll examine different kinase families, their specific substrates, and the regulatory processes governing their activity. Further, we’ll discuss the implications of kinase malfunction in disease and the development of kinase inhibitors as targeted therapies.
Kinases: The Phosphate-Adding Enzymes: Enzyme That Adds Phosphate
Kinases are a crucial class of enzymes that catalyze the transfer of a phosphate group from a high-energy donor molecule, typically ATP, to a substrate molecule. This process, known as phosphorylation, plays a pivotal role in regulating a vast array of cellular processes, from metabolism and cell signaling to gene expression and cell growth. Understanding kinases is fundamental to comprehending the intricate mechanisms that govern life at the molecular level.
Introduction to Kinases
Kinase enzymes are characterized by their ability to transfer phosphate groups, fundamentally altering the structure and function of their target molecules. Phosphate groups, consisting of a phosphorus atom bonded to four oxygen atoms, carry a negative charge. This charge significantly impacts the protein’s conformation and interactions, often acting as a molecular switch, activating or inhibiting the target protein’s activity.
Kinases exhibit remarkable substrate specificity, targeting distinct proteins or molecules based on their unique structural features. This specificity allows for precise control over diverse cellular processes. Several families of kinases exist, each exhibiting unique substrate preferences and biological functions.
| Kinase Family | Substrate Type | Biological Function |
|---|---|---|
| Serine/Threonine Kinases | Serine or threonine residues on proteins | Regulation of cell growth, metabolism, and signaling |
| Tyrosine Kinases | Tyrosine residues on proteins | Regulation of cell growth, differentiation, and survival |
| Histidine Kinases | Histidine residues on proteins | Signal transduction in bacteria and archaea |
| Lipid Kinases | Lipids | Regulation of membrane structure and signaling |
Mechanisms of Phosphate Addition
The catalytic mechanism of kinase enzymes involves a multi-step process. First, the kinase enzyme binds to both ATP and its substrate protein. ATP, the primary energy currency of cells, provides the phosphate group for transfer. The enzyme then orients the substrate’s target amino acid (serine, threonine, tyrosine, or histidine) within the active site, positioning it for nucleophilic attack on the gamma-phosphate of ATP.
This attack results in the transfer of the phosphate group to the substrate, forming a phospho-amino acid and releasing ADP. The conformational changes involved in substrate binding and catalysis are crucial for efficient phosphate transfer. The enzyme undergoes a conformational shift upon substrate binding, creating an optimal environment for the reaction. Following phosphate transfer, the enzyme releases both the phosphorylated substrate and ADP, returning to its original conformation, ready to catalyze another reaction.
Research into enzymes that add phosphate groups to proteins is ongoing, with significant implications for various biological processes. Understanding their function is crucial, and recent findings are being analyzed by sources such as drudgereport.com , which often covers breaking scientific news. Further investigation into these enzymes promises to unlock new therapeutic targets and advancements in biotechnology.
- Substrate and ATP bind to the kinase.
- The enzyme orients the substrate’s target amino acid near the gamma-phosphate of ATP.
- The target amino acid attacks the gamma-phosphate, forming a phospho-amino acid.
- ADP is released.
- The phosphorylated substrate is released.
Specific Examples of Phosphate-Adding Enzymes
Several enzymes exemplify the diverse roles of phosphate-adding enzymes.
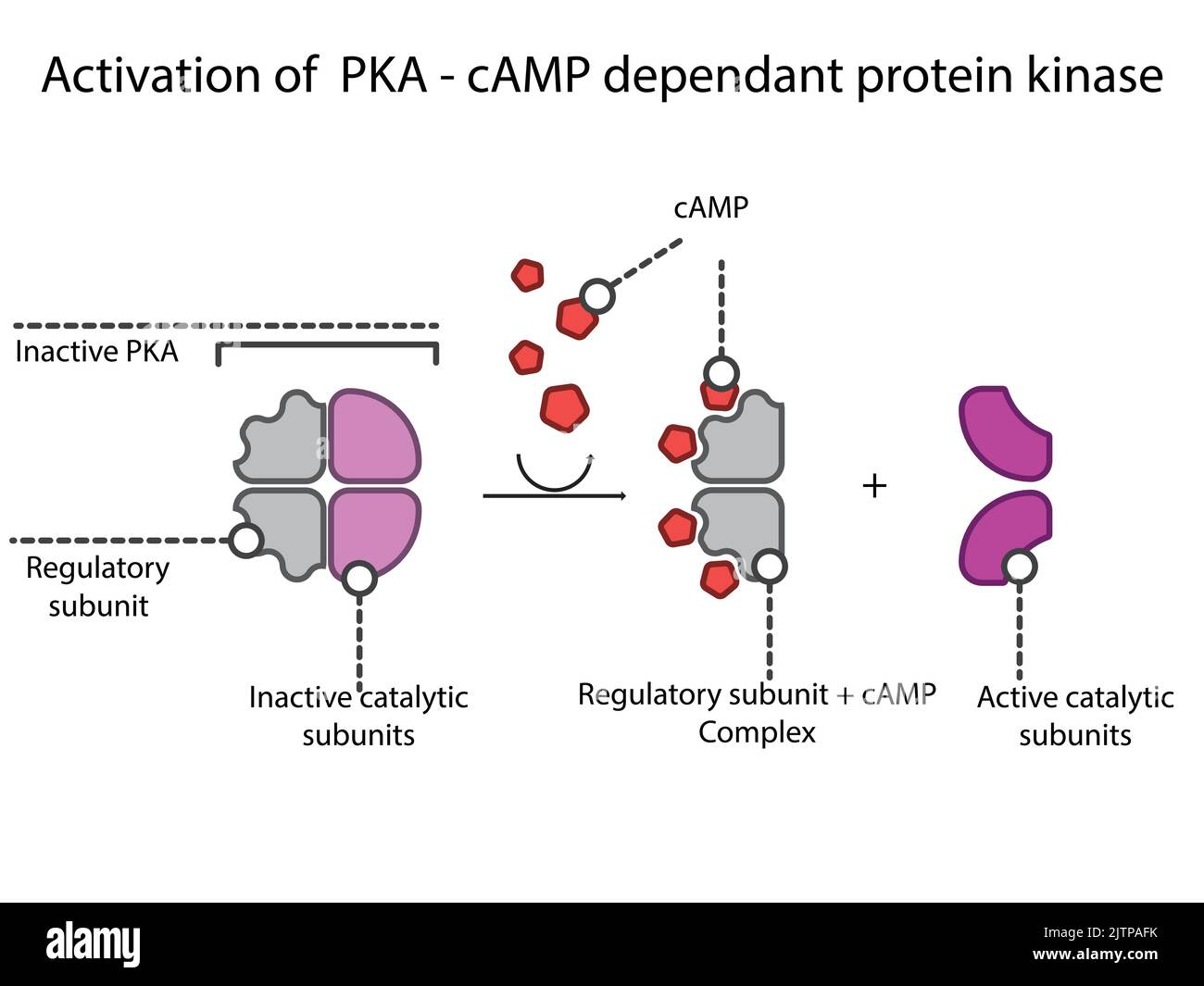
- Protein Kinase A (PKA):
- Substrate: Serine and threonine residues on various proteins.
- Structure: A tetrameric enzyme composed of two regulatory and two catalytic subunits.
- Function: Involved in diverse cellular processes, including metabolism, gene expression, and cell growth.
- Catalytic Mechanism: Follows the general kinase mechanism, utilizing ATP to transfer a phosphate group to serine or threonine residues.
- Tyrosine Kinase Receptor (RTK):
- Substrate: Tyrosine residues on proteins.
- Structure: Transmembrane receptor with an extracellular ligand-binding domain and an intracellular kinase domain.
- Function: Plays crucial roles in cell growth, differentiation, and survival, often acting as receptors for growth factors.
- Catalytic Mechanism: Ligand binding induces dimerization and autophosphorylation, activating the kinase domain to phosphorylate other target proteins.
- Phosphoinositide 3-Kinase (PI3K):
- Substrate: Phosphoinositides, membrane lipids.
- Structure: Heterodimeric enzyme composed of a regulatory and a catalytic subunit.
- Function: Regulates various cellular processes, including cell growth, survival, and metabolism, often acting downstream of RTKs.
- Catalytic Mechanism: Utilizes ATP to phosphorylate inositol rings on phosphoinositides, generating second messengers that activate downstream signaling pathways.
Regulation of Kinase Activity
Kinase activity is tightly regulated to ensure precise control over cellular processes. Allosteric regulation involves the binding of molecules to sites other than the active site, causing conformational changes that either activate or inhibit kinase activity. Post-translational modifications, such as phosphorylation, ubiquitination, and acetylation, can also modulate kinase activity. Many signaling pathways rely on the sequential activation of multiple kinases, forming kinase cascades that amplify and diversify cellular signals.
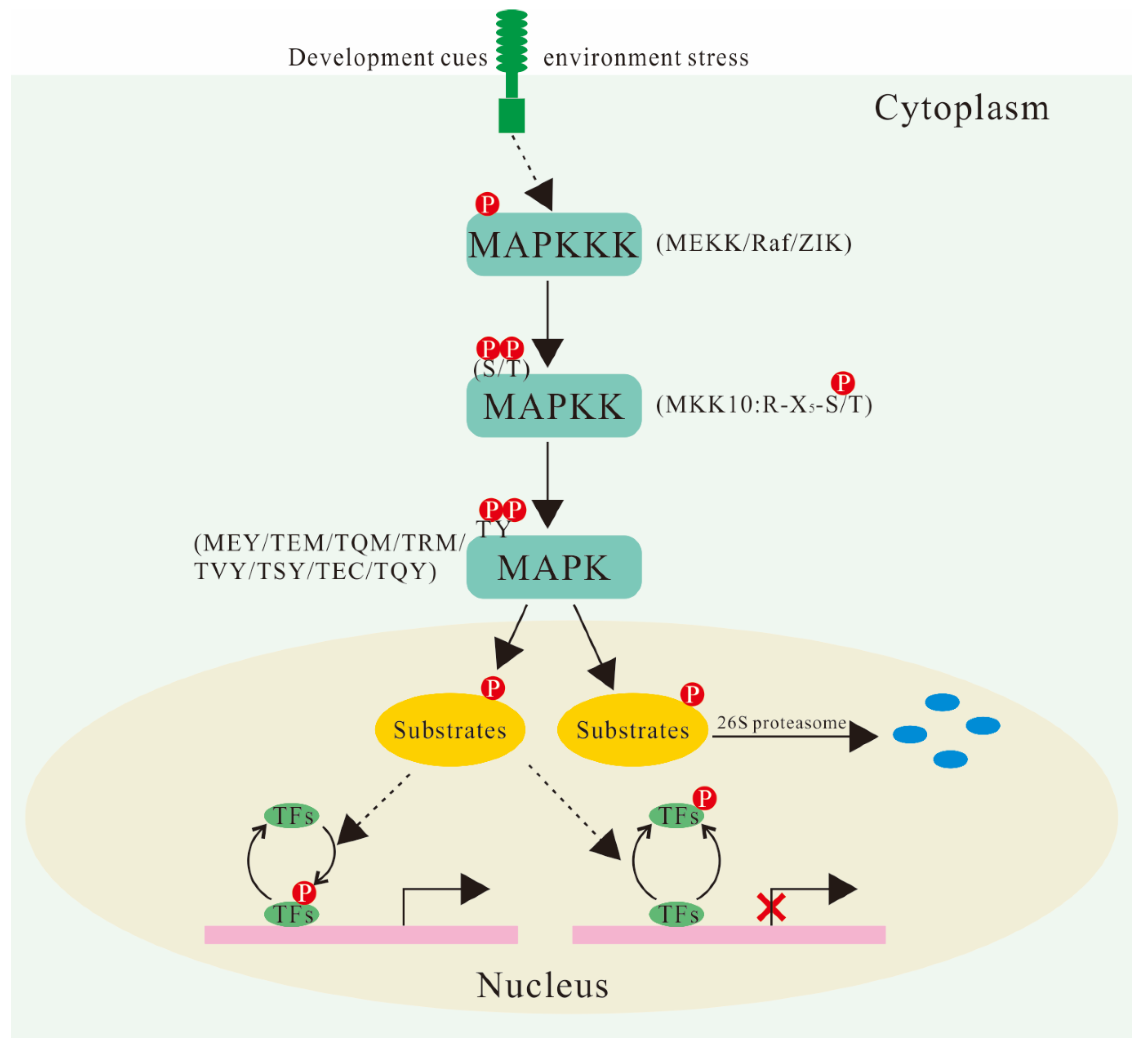
For example, the MAPK pathway, a crucial signaling pathway involved in cell growth and differentiation, involves a cascade of three kinases: MAPKKK, MAPKK, and MAPK.
The Impact of Kinase Dysfunction, Enzyme That Adds Phosphate
Source: alamy.com
Malfunctioning kinases can have profound consequences, leading to various diseases. Kinase dysregulation is implicated in a wide range of cancers, where uncontrolled cell growth and proliferation are hallmarks of the disease. Other diseases associated with kinase dysfunction include autoimmune disorders, neurodegenerative diseases, and metabolic disorders.
- Chronic Myelogenous Leukemia (CML): Caused by a chromosomal translocation that creates a constitutively active fusion kinase, BCR-ABL.
- Non-small cell lung cancer: Frequently involves mutations in EGFR (epidermal growth factor receptor), a tyrosine kinase.
Kinase inhibitors are increasingly used as therapeutic agents, targeting specific kinases involved in disease pathogenesis.
Applications of Kinase Research
Kinase research has significant implications for medicine and biotechnology. Kinase inhibitors are valuable therapeutic agents, particularly in cancer treatment. They selectively target specific kinases involved in cancer development and progression.
| Drug Name | Target Kinase | Disease Indication |
|---|---|---|
| Imatinib | BCR-ABL | Chronic Myelogenous Leukemia (CML) |
| Gefitinib | EGFR | Non-small cell lung cancer |
| Trastuzumab | HER2 | Breast cancer |
| Vemurafenib | BRAF | Melanoma |
Epilogue
Source: mdpi-res.com
The study of enzymes that add phosphate groups—kinases—reveals a complex and finely tuned system vital for cellular regulation and overall organismal health. From the intricate mechanisms of phosphorylation to the critical role kinases play in disease, research in this field continues to illuminate the fundamental processes of life and provide avenues for innovative therapeutic interventions. Further investigation into the specificities of different kinase families promises to unlock even more targeted treatments for a range of debilitating conditions.
